
— Clinical Trials —
Clinical Operations and
SaaS Solutions

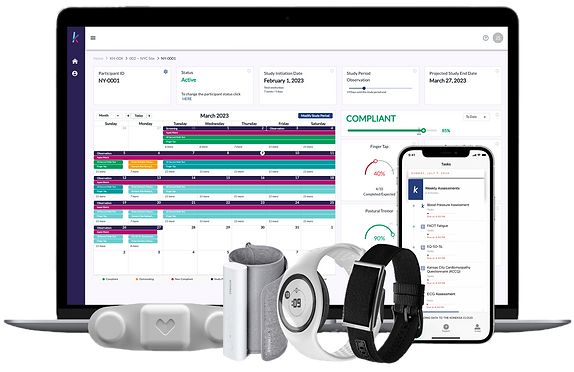
Koneksa supports the full lifecycle of digital clinical trials -
with a focus on validated digital biomarkers and electronic clinical outcome assessments (eCOAs) and using these measures to enhance analog clinical trials.
Data Insights

Koneksa
Mobile App and Platform
Flexible Study Design
Turnkey Device Integration
Robust Algorithm Engine
Data Capture

Rotation
Flow Volume
Superficial
Temp
Heart/ SpO2
Screen Touch
Movement
Wearables +
Devices


Mobile Assessments +
ePROs


We provide scientific and operational services—
powered by our patented software platform and patient-centric app to support trials in over 55 countries.
Our platform enables the capture, transformation, and analysis of high-frequency health data to generate clinical-grade insights remotely.

Proven Performance
Trusted Partnerships
Clinical
Deployments
Abstracts, Patents and Publications
What We Deliver
Study Design & Advisory
Scientific advisory boards, KOL input, endpoint selection, feasibility analysis, and early concept design.
Execution Services
ePRO translation, IRB submissions, device kitting, training, and 24/7 multilingual support.
Supporting Studies
Pilot, validation, usability, and retrospective studies to support endpoint development.
Device Integration
Evaluation, qualification, provisioning, and integration into the Koneksa platform for study use.
Data Modeling & Insights
Proprietary algorithms and model development spanning neuro, cardio, respiratory, rare diseases and more.
Clinical Endpoint Development
Integrated Services to Advance Digital Trial Success
Koneksa delivers expert support across the full lifecycle of endpoint development —
from portfolio prioritization to final execution. Our cross-functional services are designed to close the gap between clinical need and validated digital evidence.
Trusted by
and more

Delivering Digital Innovation Across Diverse Clinical Research Programs
At Koneksa, we’ve supported a wide range of clinical studies across leading pharmaceutical, biotech, and academic institutions. Our experience spans multiple therapeutic areas with a strong track record in deploying digital measures in both interventional and observational trials.

Therapeutic Area Expertise
Koneksa is advancing clinical research by developing, validating, and deploying digital biomarkers. Check out our clinical pipeline by therapeutic area and see for yourself.
Parkinson’s disease, multiple sclerosis, and other CNS indications with mobile assessments, voice analysis, and wearable-based motor tracking
Cardiovascular
Digital data integration in heart failure and cardiomyopathies
Real-world monitoring in asthma and COPD, including inhaler adherence and breathing pattern analysis
Remote patient monitoring during and after treatment for COVID-19 and similar conditions
Digital solutions for capturing disease-specific symptoms in addition to physical activity, sleep, and vital signs
Whether running decentralized studies, integrating novel digital biomarkers, or validating endpoints in rare or complex diseases, our team brings proven expertise in study design, digital tool deployment, and longitudinal data collection at scale.

Types of Studies Supported
Phase 1–3 Interventional Trials
Longitudinal, Observational, and
Natural History Studies
| Neurology
Over a third of our portfolio to date has focused on passive and active digital measures of neurological symptoms, including nine Phase 2/3 trials.
Angelman Syndrome
COMPLETE
Chronic Central Pain
COMPLETE
Friedreich Ataxia
COMPLETE
Glucose Transporter Type 1 Deficiency Syndrome
COMPLETE
Multiple Sclerosis A
COMPLETE
Multiple Sclerosis B
COMPLETE
Narcolepsy A
COMPLETE
Narcolepsy B
COMPLETE
Parkinson's Disease A
COMPLETE
Parkinson's Disease B
CLOSE-OUT
Multiple Sclerosis C
ACTIVE
Multiple Sclerosis D
ACTIVE
Parkinson's Disease C
ACTIVE
Parkinson's Disease D
ACTIVE
Parkinson's Disease E
ACTIVE
Parkinson's Disease F
ACTIVE
Parkinson's Disease G
FORCASTED + STARTUP
| Respiratory
We have experience conducting Phase 1-4 trials focused on evaluating treatments for respiratory conditions such as asthma and COPD.
Asthma
COMPLETE
Asthma
COMPLETE
Asthma
COMPLETE
Asthma
COMPLETE
Chronic Obstructive Pulmonary Disease A
COMPLETE
Chronic Obstructive Pulmonary Disease B
COMPLETE
Idiopathic Pulmonary Fibrosis
COMPLETE
| Oncology
Our work in oncology has supported interventional trials and observational studies, both academic and industry-sponsored, to evaluate activities of daily living during cancer treatment.
Lung Cancer A
COMPLETE
Multiple Myeloma
COMPLETE
Solid Tumor
COMPLETE
Lung Cancer B
ACTIVE
| Infectious Disease
The onset of the COVID-19 pandemic required extensive scientific research during a challenging time; we responded by supporting our clients with creative solutions for measuring activity, sleep, and vital signs in the home setting.
Covid-19 A
COMPLETE
Covid-19 B
COMPLETE
Covid-19 C
CLOSE-OUT
| Musculoskeletal Conditions
Much of our work in musculoskeletal conditions, both adult and pediatric, has required the development and evaluation of novel digital measures.
Achondroplasia
COMPLETE
Skeletal Disorder
CLOSE-OUT
Total Hip Replacement Candidate
CLOSE-OUT
| Other Conditions
We have expertise and experience working in many therapeutic areas, providing digital solutions for capturing disease-specific symptoms in addition to physical activity, sleep, and vital signs.
Hemophilia
COMPLETE
Paroxysmal Nocturnal Hemoglobinuria
COMPLETE
Schizophrenia A
COMPLETE
Schizophrenia B
COMPLETE
Atopic Dermititis
COMPLETE
Cat Allergy
COMPLETE
Hepatic Insufficiency
COMPLETE
Dilated Cardiomyopathy
CLOSE-OUT
| Measure Evaluations Healthy Controls
Much of our early measure development work has been conducted amongst healthy adults, in addition to the development of datasets for use as healthy control comparators.
Healthy A
COMPLETE
Healthy B
COMPLETE
Healthy C
COMPLETE
Healthy D
COMPLETE
Healthy E
COMPLETE
Healthy F
COMPLETE
Healthy G
COMPLETE
Healthy H
COMPLETE
Digital and Analog Measures and Capabilities Used in Our Clinical Programs
Koneksa’s platform supports the deployment of a wide range of digital endpoints, including:
Wearable
sensor data
(e.g., tremor, gait, ECG, activity)
Mobile, cognitive
and motor
function tasks
Speech
and voice biomarkers
Electronic
PROs and
symptom diaries
In-clinic and remote clinician-assessed video capture
Continuous compliance
and adherence monitoring
